Ocular Therapeutix™ Reports Positive Results from Landmark SOL-1 Phase 3 Superiority Trial in Wet AMD
First ever successful demonstration of superiority in an FDA-aligned wet AMD trial comparing a novel investigative agent to an approved anti-VEGF treatment
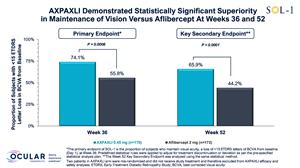
Superiority primary endpoint met with 74.1% of subjects in the AXPAXLI™ arm maintaining vision at Week 36, a 17.5% risk difference (p=0.0006), compared to aflibercept (2 mg) arm
65.9% of subjects treated with AXPAXLI maintained vision at Week 52, a 21.1% risk difference (p<0.0001), compared to aflibercept (2 mg) arm
Rescue-free rates in the AXPAXLI arm were 80.6%, 74.7%, and 68.8% at Weeks 24, 36, and 52
55.9% of subjects treated with AXPAXLI maintained CSFT within 30 μm from baseline at Week 36, a 17.1% risk difference (nominal p=0.0013) compared to aflibercept (2 mg) subjects
77.1% of AXPAXLI subjects randomized in SOL-1 would have been rescue-free at Week 24 using SOL-R rescue criteria which align closely with clinical practice
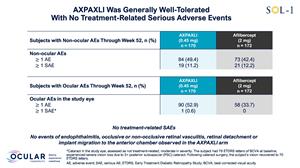
AXPAXLI was generally well-tolerated in SOL-1 with no treatment-related ocular SAEs
Subjects have been re-dosed at Week 52
Ocular plans to submit New Drug Application (NDA) based on SOL-1 data, subject to planned formal discussions with U.S. FDA
Conference call and webcast today, February 17, 2026, at 8:00 AM ET, to discuss topline data
Detailed data to be presented at 49th Macula Society Annual Meeting
BEDFORD, Mass., Feb. 17, 2026 (GLOBE NEWSWIRE) -- Ocular Therapeutix, Inc. (NASDAQ: OCUL, “Ocular”), an integrated biopharmaceutical company committed to redefining the retina experience, today announced positive topline results from the SOL-1 Phase 3 superiority trial of AXPAXLI (also known as OTX-TKI), its investigational product candidate, for the treatment of wet age-related macular degeneration (AMD). After a loading phase, the SOL-1 trial compared a single dose of AXPAXLI (0.45 mg) to a single dose of aflibercept (2 mg) in patients with wet AMD. Under the pre-specified statistical analysis plan, which is aligned with the U.S. FDA in the SOL-1 Special Protocol Assessment (SPA) agreement, the superiority primary endpoint at Week 36 was met with high statistical significance (p=0.0006). In addition to achieving the primary endpoint, AXPAXLI showed either statistical significance or numerical superiority to aflibercept (2 mg) in key secondary and prespecified exploratory endpoints.
“We are thrilled to report today’s historic data that position AXPAXLI to potentially become one of the most consequential advances in retina. SOL-1 data provide robust evidence that support AXPAXLI’s potential to deliver safe, durable, and clinically significant visual and anatomic outcomes with a meaningfully reduced treatment burden,” said Pravin U. Dugel, MD, Executive Chairman, President and Chief Executive Officer of Ocular Therapeutix. “Demonstrating superiority in wet AMD to a single dose of aflibercept (2 mg) under the FDA’s stringent evidentiary standard required in this trial was an exceptionally difficult bar. Many pivotal programs have failed at demonstrating superiority in a Phase 3 trial, and we do not foresee other programs attempting this challenge or pursuing a superiority label in this indication. In addition to reaching the primary endpoint, approximately two-thirds of AXPAXLI subjects were rescue free through Week 52. The fact that AXPAXLI delivered these consistent, durable, and superior outcomes in this trial provides further evidence of axitinib’s potency and pan-VEGF suppression paired with the robustness and safety of our proprietary hydrogel platform.”
“The SOL-1 results mark one of the most important advances in the treatment of wet AMD since the advent of anti-VEGF biologics 20 years ago. AXPAXLI is the first and only drug with a novel mechanism to successfully demonstrate superiority to an approved anti-VEGF treatment in an FDA-aligned study for wet AMD,” commented Arshad M. Khanani, MD, MA, FASRS, Director of Clinical Research at Sierra Eye Associates in Reno, Nevada, and Steering Committee Chair for the SOL Program. “To see sustained anatomic responses with 55.9% of AXPAXLI subjects maintaining CSFT within 30 microns at Week 36 highlights the fluid control and stability we hope to see in everyday practice. In addition, two-thirds of subjects remain rescue-free at Week 52 suggesting this could potentially be an annual treatment in many patients. These data show AXPAXLI’s potential to significantly reduce treatment burden and optimize long-term visual outcomes for our patients and, if approved, should support rapid and broad clinical adoption.”
SOL-1 Topline Results Summary
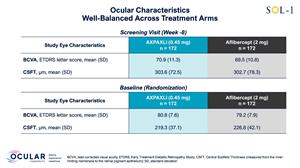
SOL-1 is a superiority trial being conducted under a Special Protocol Assessment (SPA) agreement with the U.S. FDA. For the primary endpoint, the trial evaluated a single injection of AXPAXLI versus a single aflibercept (2 mg) injection in 344 newly-diagnosed wet AMD subjects randomized after an initial 8-week loading segment. Subjects were randomized in SOL-1 after showing a peak response to two aflibercept (2 mg) injections, reaching approximately 20/20 vision or experiencing an improvement of at least 10 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters of best corrected visual acuity (BCVA), in addition to satisfying other criteria. This is a population that was specifically selected to lose vision. Baseline characteristics were well-balanced between the AXPAXLI and aflibercept (2 mg) arms. Although the primary endpoint was at Week 36, the trial remained masked through the Week 52 durability assessment. At Week 52, subjects were re-dosed with their respective initial treatments of AXPAXLI or aflibercept (2 mg). Per the protocol, subjects will be re-dosed again at Week 76 and will be followed on a masked-basis for safety until the end of Week 104.
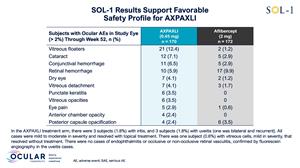
Safety Overview
As of the Week 52 database lock on February 5, 2026, AXPAXLI was generally well-tolerated in SOL-1 with no observed treatment-related ocular or systemic serious adverse events (SAEs). There were no events of endophthalmitis, occlusive or non-occlusive retinal vasculitis, retinal detachment, or implant migration to the anterior chamber observed in the AXPAXLI arm.
For additional detailed safety information, see Figure 2 and Figure 3.
Week 36 Primary Endpoint
The primary endpoint of SOL-1 is the proportion of subjects who maintain visual acuity, a loss of <15 ETDRS letters of BCVA from baseline (Day 1), at Week 36. Predefined statistical rules were applied to adjust for treatment discontinuation or deviation as per the pre-specified statistical analysis plan. The SOL-1 primary endpoint was designed to support a potential superiority label in wet AMD based on the FDA’s 2023 draft guidance for wet AMD therapies and was agreed to by the FDA in the Company’s SPA agreement.
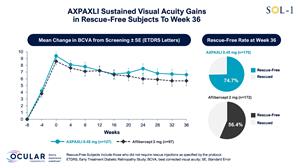
AXPAXLI met its primary endpoint at Week 36 with high statistical significance. The proportion of subjects with <15 ETDRS letters lost from baseline at Week 36 was 74.1% in the AXPAXLI arm compared to 55.8% in the aflibercept (2 mg) arm, with a risk difference of 17.5% and p-value of 0.0006 per the pre-specified statistical model, and with an observed difference of 18.3%. The risk difference is the difference in the probability of maintaining vision in the treatment arm compared to the control arm as per the pre-specified statistical model. The observed difference is the numerical difference of the observed event rate between the two arms. Multiple sensitivity analyses for the primary endpoint were also statistically significant and confirm that the data are robust.
Week 52 Durability Assessment
As a pre-specified key secondary endpoint, Ocular evaluated the proportion of subjects who maintained visual acuity at Week 52 using the same analysis as the primary endpoint.
AXPAXLI met its Week 52 durability assessment with high statistical significance. The proportion of subjects who maintained vision at Week 52 was 65.9% in the AXPAXLI arm compared to 44.2% in the aflibercept (2 mg) arm, with a risk difference of 21.1% and a p-value of <0.0001 per the pre-specified statistical model compared to aflibercept (2 mg) subjects, and with an observed difference of 21.7%.
On Protocol Rescue-Free Rates
As a pre-specified exploratory endpoint, Ocular evaluated the proportion of patients who did not require rescue injections as specified by the protocol rescue criteria, which includes a BCVA loss of ≥15 ETDRS letters from baseline or new vision-threatening macular hemorrhage.
The rescue-free rates in the AXPAXLI arm were 80.6%, 74.7%, and 68.8% at Weeks 24, 36, and 52, respectively, compared to 72.1%, 56.4%, and 47.7% for the aflibercept (2 mg) arm at the same time periods. The observed differences were 8.5%, 18.3%, and 21.1% in favor of the AXPAXLI arm at Weeks 24, 36, and 52, respectively.
Fluid Control
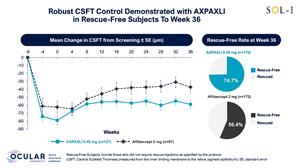
As a pre-specified exploratory endpoint, Ocular evaluated the proportion of subjects maintaining Central Subfield Thickness (CSFT) within 30 μm from baseline.
At Weeks 36 and 52, subjects in the AXPAXLI arm demonstrated superior and sustained CSFT control as compared to subjects in the aflibercept (2 mg) arm. Detailed data for the pre-specified exploratory endpoint are shown below:
- Week 36: 55.9% of subjects treated with AXPAXLI maintained CSFT within 30 μm from baseline versus 37.8% of subjects in the aflibercept (2 mg) arm, representing a risk difference of 17.1% in favor of AXPAXLI (nominal p=0.0013).
-
Week 52: 44.1% of subjects treated with AXPAXLI maintained CSFT within 30 μm from baseline versus 34.9% of subjects in the aflibercept (2 mg) arm, representing a risk difference of 8.4% in favor of AXPAXLI (nominal p=0.1094).
Darius M. Moshfeghi, Chief of the Retina Division at Byers Eye Institute of Stanford University School of Medicine noted, “Congratulations to Ocular on successfully executing a challenging superiority trial that other product candidates will find incredibly difficult to replicate. When I look at these data, I see a product candidate in AXPAXLI that shows a level of disease control and durability that stands apart from what we have historically experienced in wet AMD. The durability is compelling, with 74.1% of subjects maintaining vision through the Week 36 primary endpoint, and an impressive 65.9% maintaining vision through Week 52. The durability demonstrated in SOL-1 meaningfully exceeds what we typically expect from a single treatment. Importantly, fewer rescues and a longer time to first rescue than what we see in clinical practice today should translate into fewer urgent interventions and more predictable disease control. The extended duration of disease control observed in SOL-1 would provide meaningful coverage if a patient misses a visit, which is a very real issue in clinical practice, especially in this patient population. Taken together, these data provide substantial evidence of AXPAXLI’s potential to transform how we manage wet AMD.”
SOL-1 Rescue Free Analysis Using SOL-R Rescue Criteria
The SOL-1 trial, including its primary endpoint, was aligned with the FDA through the Company’s SPA agreement with the goal of achieving a superiority label for AXPAXLI. The Company is also conducting a second non-inferiority registrational trial, SOL-R. The protocol rescue criteria in SOL-R of >5 ETDRS letter loss in BCVA plus a ≥75 μm increase in CSFT are designed to track more closely with real-world clinical practice as compared to the rescue criteria used in SOL-1.
In a prespecified exploratory analysis applying the SOL-R protocol rescue criteria to the SOL-1 results, AXPAXLI demonstrated a 77.1% rescue-free rate at Week 24. Week 24 was selected as the timepoint for this analysis to align with the re-dosing interval being used in the SOL-R trial.
Nadia K. Waheed, MD, MPH, Chief Medical Officer of Ocular Therapeutix, commented, “The SOL-1 data give us great confidence in the SOL-R non-inferiority study, which is being conducted in a population likely to be more stable and easier to control than SOL-1. SOL-R was intentionally designed to complement SOL-1 and further validate AXPAXLI’s durability in a six-month re-dosing paradigm. If we were to apply the more clinically relevant SOL-R rescue criteria to SOL-1, an impressive 77.1% of AXPAXLI patients would have remained rescue-free through the six-month re-dosing window. We expect these rescue-free rates to be even higher in populations such as the one randomized in SOL-R, given SOL-R’s patient enrichment strategy over the six months prior to randomization that excluded subjects with early persistent fluid or significant fluid fluctuations. Most importantly, we continue to be encouraged by AXPAXLI’s safety profile in SOL-1. Patients have now been re-dosed, and retention continues to be exceptional. Ultimately, we believe the implications of the SOL-1 trial results extend far beyond clinical endpoints. These data provide strong evidence of AXPAXLI’s potential to dramatically reduce treatment burden while maintaining vision, which should translate into improved quality of life for patients and caregivers, markedly less complicated logistics for physicians and their practices, and the clear value of a simplified treatment paradigm for payors.”
Next Steps
Detailed results from the SOL-1 trial will be presented at the 49th Macula Society Annual Meeting, taking place between February 25 - 28, 2026.
SOL-1 subjects remain masked and have received their second injection of their respective initial treatment at their Week 52 visit. Subjects will receive another injection of their respective initial treatment at Week 76 and will be followed on a masked-basis for safety until the end of Week 104.
Ocular intends to submit a New Drug Application (NDA) based on SOL-1 data, subject to planned formal discussions with the U.S. FDA. If approved, AXPAXLI could be the first tyrosine kinase inhibitor (TKI) to be commercialized in wet AMD, and potentially the only therapy with a superiority label and best-in-disease durability. The complementary SOL-R Phase 3 non-inferiority trial of AXPAXLI in wet AMD will continue as planned, with topline data expected in 1Q 2027.
SOL-1 Topline Data Conference Call and Webcast Information:
Ocular Therapeutix will host a conference call and webcast today, Tuesday, February 17, 2026, at 8:00 AM ET to discuss the SOL-1 topline results. To register for and access the webcast, please click here. The live and archived webcast can also be accessed by visiting the Ocular Therapeutix website on the Events and Presentations section of the Investor Relations page. A replay of the webcast will be archived for at least 30 days.
About AXPAXLI
AXPAXLI™ (also known as OTX-TKI) is an investigational, bioresorbable, intravitreal hydrogel incorporating axitinib, a small molecule, multi-target, tyrosine kinase inhibitor with anti-angiogenic properties, being evaluated for the treatment of wet AMD and diabetic retinal disease.
About the SOL-1 Trial
The registrational Phase 3 SOL-1 trial (NCT06223958) is designed to evaluate the safety and efficacy of AXPAXLI in a multi-center, double-masked, randomized (1:1), parallel group trial that involves more than 100 clinical trial sites located in the U.S. and Argentina. In December 2024, the trial completed randomization of 344 treatment-naïve subjects with a diagnosis of wet AMD in the study eye. Two randomized subjects withdrew from the trial prior to receiving Day 1 treatment.
The superiority trial has an eight-week loading segment prior to randomization. During the loading segment, subjects who have 20/80 vision or better and a central subfield thickness (CSFT) of ≤500 μm receive two doses of aflibercept (2 mg) at Week -8 and Week -4. Subjects who achieve best corrected visual acuity (BCVA) of 20/20 at Day 1 (baseline) or gain at least 10 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at Day 1 along with a CSFT of ≤350 μm were then randomized to receive a single dose of AXPAXLI or a single dose of aflibercept (2 mg). At Week 52 and at Week 76, all subjects are re-dosed with their respective initial treatment of AXPAXLI (0.45 mg) or aflibercept (2 mg). Subjects will be followed for safety until the end of Week 104.
Throughout the trial, subjects are assessed monthly. Trial subjects and designated trial personnel will remain masked through the end of Week 104. The clinical trial protocol requires that, during the trial, subjects in either arm meeting the pre-specified rescue criteria, which includes a BCVA loss of ≥15 ETDRS letters from baseline or new vision-threatening macular hemorrhage, will receive a supplemental dose of aflibercept (2 mg). The protocol provides that after the first rescue injection, rescue therapy may be provided at investigator discretion per their clinical judgement.
The primary endpoint of SOL-1 is the proportion of subjects who maintain visual acuity, a loss of <15 ETDRS letters of BCVA from baseline, at Week 36. Predefined statistical rules were applied to adjust for treatment discontinuation or deviation as per the pre-specified statistical analysis plan. The trial remained masked following Week 36 and subjects were evaluated for treatment durability at Week 52. The trial is being conducted under a Special Protocol Assessment (SPA) agreement with the FDA.
About the SOL-R Trial
The registrational Phase 3 SOL-R trial (NCT06495918) is designed to evaluate the safety and efficacy of AXPAXLI in a multi-center, double-masked, randomized (2:2:1), three-arm trial that includes sites located in the U.S., Argentina, India, and Australia in subjects who are treatment-naïve or were diagnosed with wet AMD in the study eye within about four months prior to enrollment. Further, to qualify for screening, a subject’s study eye must have had a BCVA ETDRS letter score of ≥34 (~20/200). In December 2025, the trial completed the randomization of 631 subjects.
This non-inferiority trial reflects a patient enrichment strategy over the six months prior to randomization that includes three screening doses of any anti-VEGF therapy, excluding brolucizumab-dbll, and monitoring to exclude those subjects with early persistent fluid or significant retinal fluid fluctuations. Subjects who continue to meet eligibility, defined as a CSFT of ≤350 μm at Week -12 and Week -8 with ≤35 μm CSFT increase from the lowest CSFT at any prior visit, entered a run-in period and received two loading doses of aflibercept (2 mg) prior to Day 1. Subjects in the first arm receive a single dose of AXPAXLI (0.45 mg) at Day 1 and are re-dosed at Weeks 24, 48, and 72. Subjects in the second arm receive aflibercept (2 mg) on Day 1 and per label every eight weeks thereafter. Subjects in the third arm receive a single dose of aflibercept (8 mg) at Day 1 and are re-dosed at Weeks 24, 48, and 72, aligned with the AXPAXLI treatment arm for adequate masking. Subjects will be followed for safety until the end of Week 96. Throughout the trial, subjects are assessed monthly. Trial subjects and designated trial personnel will remain masked through the end of Week 96. Subjects in any arm that meet pre-specified rescue criteria will receive a supplemental dose of aflibercept (2 mg). The pre-specified rescue criteria include a >5-letter loss in visual acuity plus a ≥75 μm increase in CSFT.
The primary endpoint of SOL-R is to demonstrate non-inferiority in mean BCVA change from baseline between the AXPAXLI and on-label aflibercept (2 mg) arms at Week 56. As per the protocol agreed to by the FDA, the non-inferiority margin for the lower bound is -4.5 letters of mean BCVA when compared to aflibercept (2 mg) dosed every eight weeks. In a written Type C response received in August 2024, and a subsequent written response received in December 2024, the FDA agreed that the SOL-R repeat dosing wet AMD trial, with a primary endpoint at Week 56, should be appropriate as an adequate and well-controlled trial in support of a potential New Drug Application and product label for wet AMD.
About Wet AMD
Wet age-related macular degeneration (wet AMD) is a leading cause of severe, irreversible vision loss affecting approximately 14.8 million individuals globally and 1.7 million in the United States alone. Wet AMD causes vision loss due to abnormal new blood vessel growth and hyperpermeability and associated retinal vascularity in the macula, which is primarily stimulated by local upregulation of vascular endothelial growth factor (VEGF). Without prompt and continuous treatment to control this exudative activity, patients develop irreversible vision loss. With proper treatment, patients may maintain visual function for a period of time and may temporarily regain lost vision. Challenges with current therapies include pulsatile, repeated intraocular injections, treatment-related adverse events and up to 40% patient discontinuation within one year of initiating treatment with continued disease progression. Taken together, these factors lead to undertreatment and a lack of long-term vision improvement for patients.
About Ocular Therapeutix, Inc.
Ocular Therapeutix, Inc. is an integrated biopharmaceutical company committed to redefining the retina experience. AXPAXLI™ (also known as OTX-TKI), Ocular’s investigational product candidate for retinal disease, is an axitinib intravitreal hydrogel based on its ELUTYX™ proprietary bioresorbable hydrogel-based formulation technology. AXPAXLI is currently in Phase 3 clinical trials for wet age-related macular degeneration (wet AMD) and diabetic retinal disease, including non-proliferative diabetic retinopathy (NPDR).
Ocular’s pipeline also leverages the ELUTYX technology in its commercial product DEXTENZA®, an FDA-approved corticosteroid for the treatment of ocular inflammation and pain following ophthalmic surgery in adults and pediatric patients and ocular itching associated with allergic conjunctivitis in adults and pediatric patients aged two years or older, and in its investigational product candidate OTX-TIC, which is a travoprost intracameral hydrogel that has completed a Phase 2 clinical trial for the treatment of open-angle glaucoma or ocular hypertension. Ocular is currently evaluating next steps for the OTX-TIC program.
Follow the Company on its website, LinkedIn, or X.
DEXTENZA® is a registered trademark of Ocular Therapeutix, Inc. The Ocular Therapeutix logo, AXPAXLI™, ELUTYX™, and Ocular Therapeutix™ are trademarks of Ocular Therapeutix, Inc.
Forward-Looking Statements
Any statements in this press release about future expectations, plans, and prospects for the Company, including statements regarding the development and regulatory status of the Company’s product candidates, including the Company’s intention to submit a new drug application for AXPAXLI based on data from the Company’s SOL-1 trial, subject to planned formal discussions with the FDA, the timing, design, enrollment, randomization, conduct and retention of subjects in the Company’s clinical trials, including the Company’s SOL-1 and SOL-R Phase 3 clinical trials of AXPAXLI (also known as OTX-TKI) for the treatment of wet AMD; statements regarding the potential utility or adoption, if approved, of any of the Company’s product candidates; and other statements containing the words “anticipate”, “become”, “believe”, “estimate”, “expect”, “intend”, “designed”, “goal”, “may”, “might”, “plan”, “position”, “predict”, “project”, “target”, “potential”, “will”, “would”, “could”, “should”, “continue”, and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the design, timing, conduct and outcomes of ongoing and planned clinical trials, including the SOL-R trial and the second year of the SOL-1 trial; the risk that the FDA will not agree with the Company’s interpretation of the written agreements under the Special Protocol Assessments for AXPAXLI, including for the SOL-1 trial; uncertainty as to whether the FDA will accept a new drug application for AXPAXLI on the basis of a single pivotal clinical trial, namely SOL-1; uncertainty as to the minimum clinical data required to demonstrate the safety of a proposed product candidate such as AXPAXLI, even if the FDA recognizes that only one pivotal clinical trial may be required to demonstrate efficacy; the risk that even though the FDA has agreed with the overall design of the SOL-1 trial, the FDA may not find that the data generated by the trial and submitted by the Company are sufficient to demonstrate the safety and efficacy of AXPAXLI to the degree necessary to support marketing approval for wet AMD; uncertainty as to whether the Company will be able to timely satisfy the FDA’s other requirements for regulatory approval of AXPAXLI, including the FDA’s Chemistry, Manufacturing and Control’s requirements, even if the Company can satisfy the FDA’s clinical requirements to demonstrate safety and efficacy; uncertainty as to what restrictions, if any, may be imposed on the label for AXPAXLI, if approved, pending the receipt of additional clinical data or otherwise; the risk that the FDA might not agree to the Company’s design, protocol, and statistical analysis plan of the SOL-R trial; the risk that the Company and the FDA may not agree on the registrational pathway for any of its product candidates, including AXPAXLI; uncertainty as to whether the data from earlier clinical trials will be predictive of the data of later clinical trials, particularly later clinical trials that have a different design or utilize a different formulation than the earlier trials; uncertainty as to whether preliminary or interim data from a clinical trial will be predictive of final data from such trial; uncertainties regarding the potential commercial advantages and/or position of the Company’s product candidates; availability of data from clinical trials and expectations for regulatory submissions and approvals; the Company’s scientific approach and general development progress; uncertainties inherent in estimating the Company’s cash runway, future expenses and other financial results, including its ability to fund future operations, including clinical trials; and other factors discussed in the “Risk Factors” section contained in the Company’s annual or quarterly reports on file with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this press release. The Company anticipates that subsequent events and developments may cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this press release.
Investors & Media
Ocular Therapeutix, Inc.
Bill Slattery
Vice President, Investor Relations
bslattery@ocutx.com
Figure 1: Screening and Baseline Ocular Characteristics
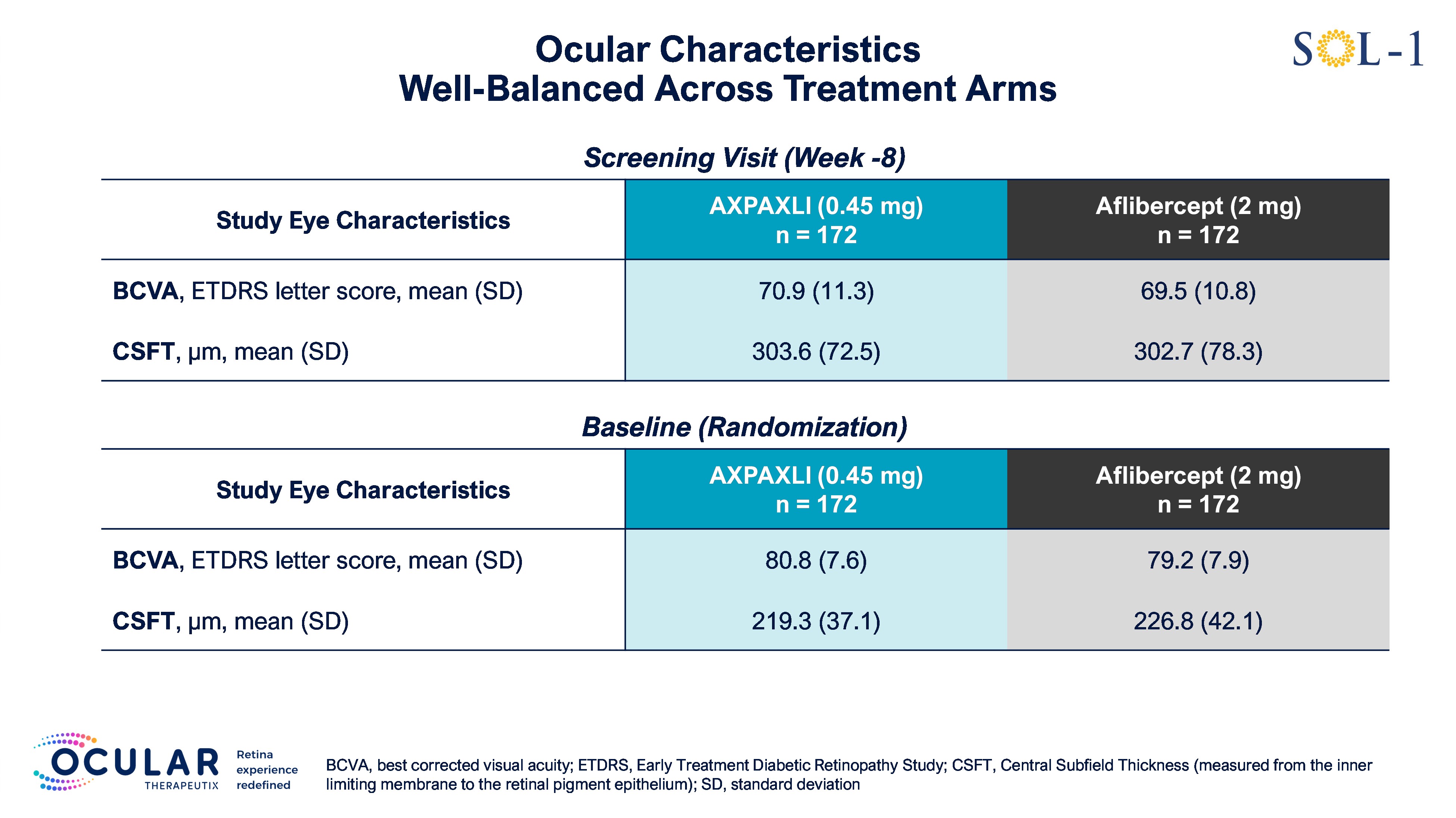
Figure 2: Safety Summary
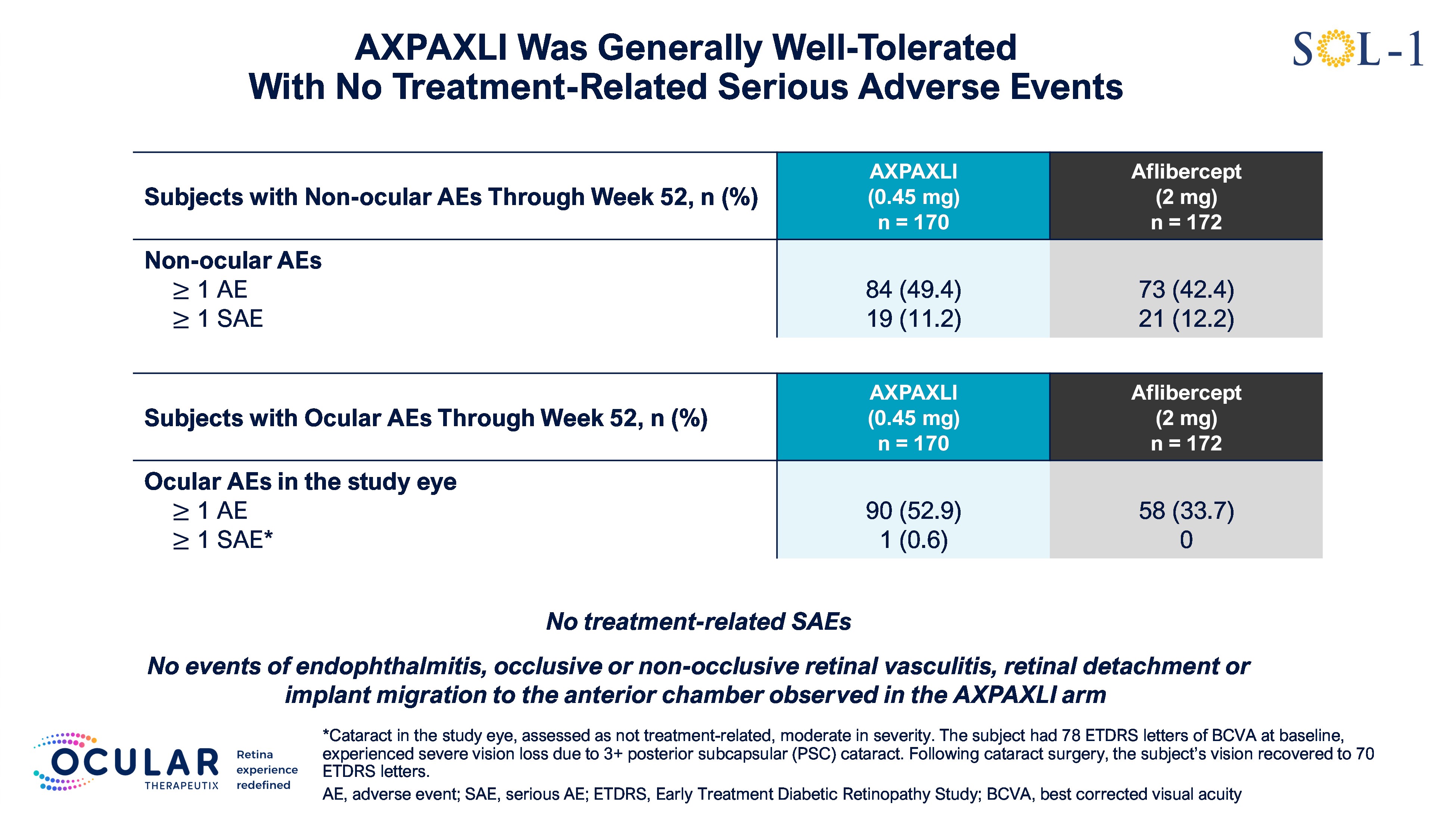
Figure 3: Additional Safety Details
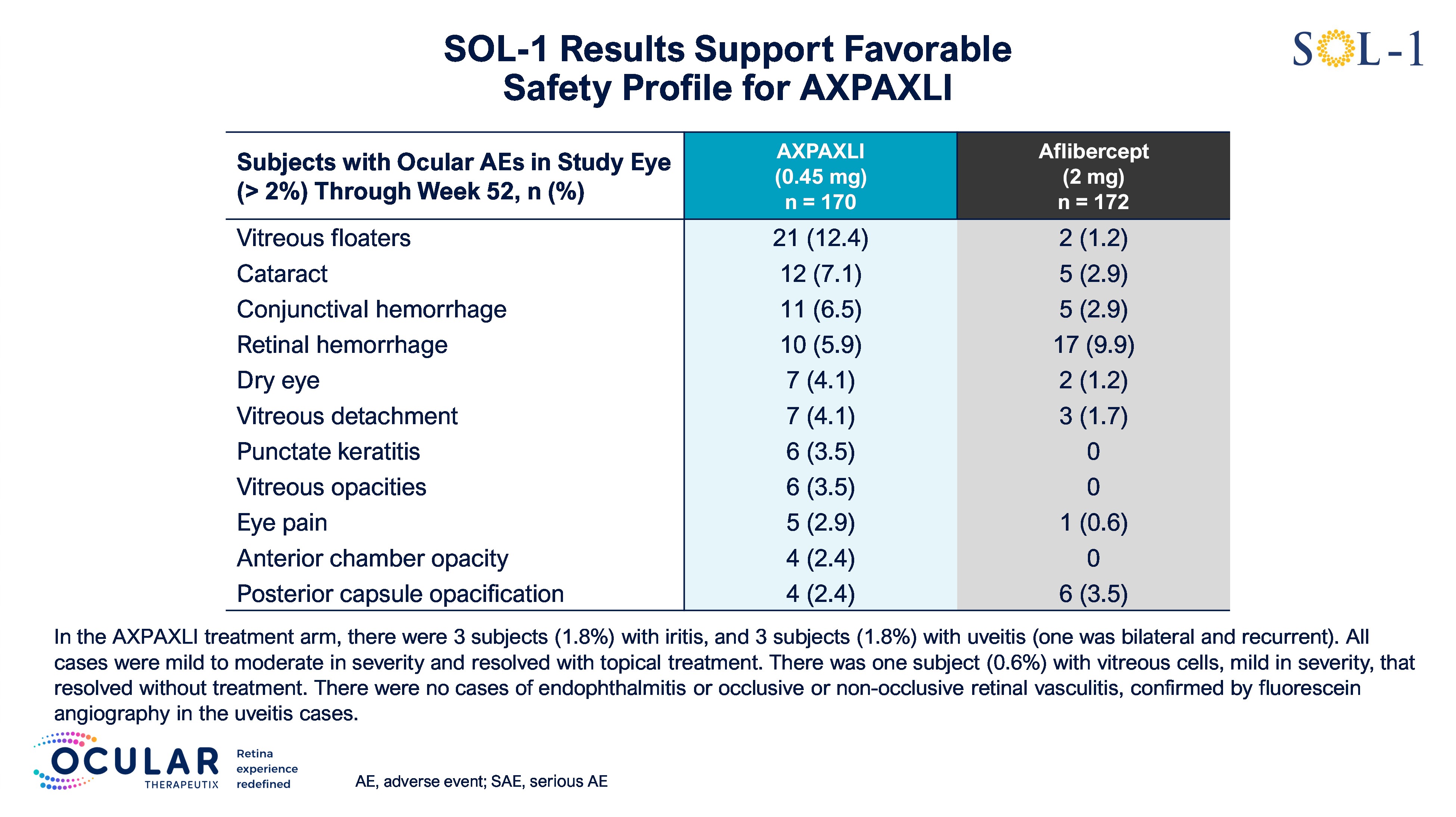
Figure 4: Proportion of Subjects with <15 ETDRS Letter Loss in BCVA from Baseline at Week 36 and Week 52
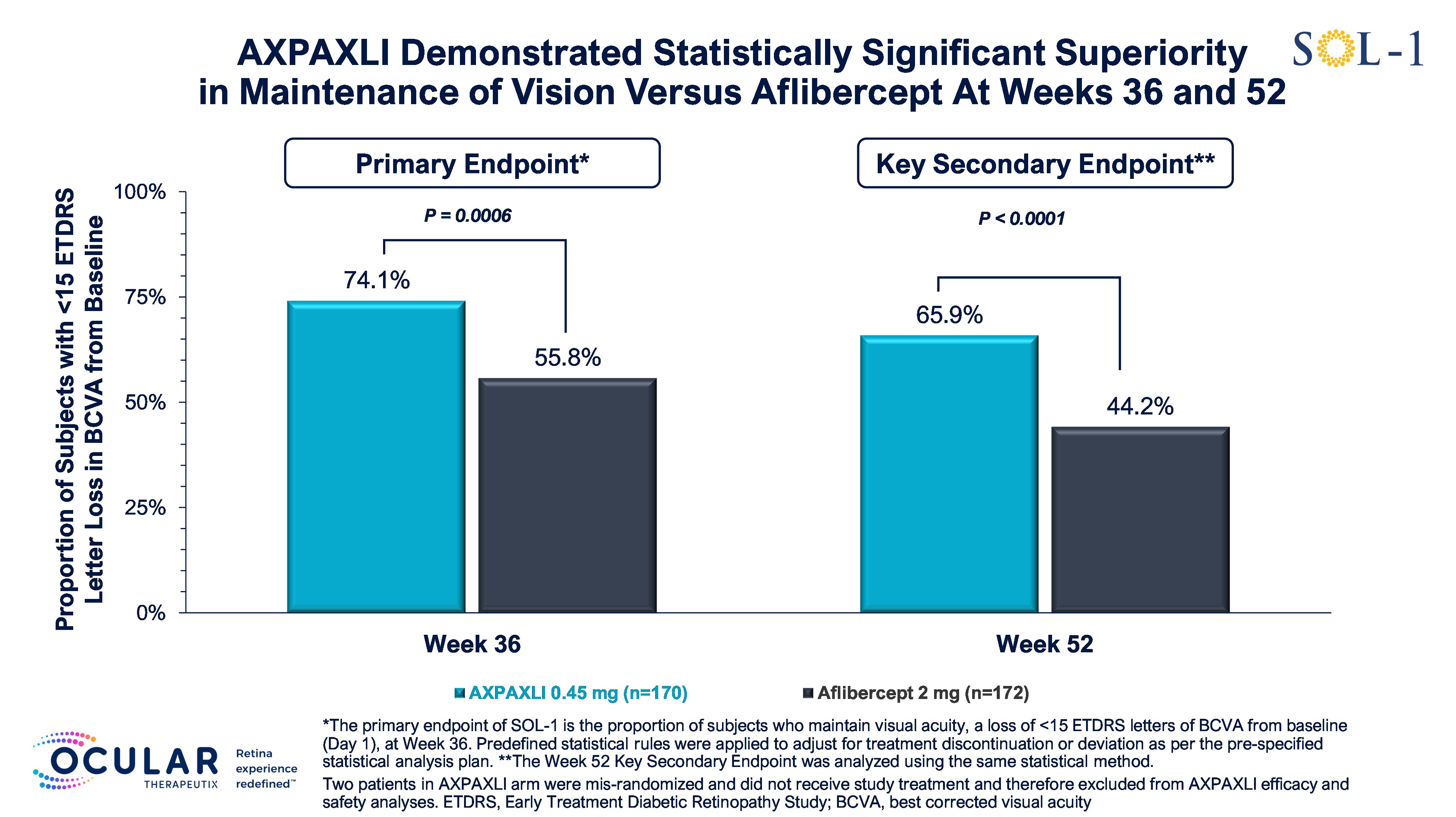
Figure 5: Mean Change in BCVA in Rescue-Free Subjects from Screening to Week 36
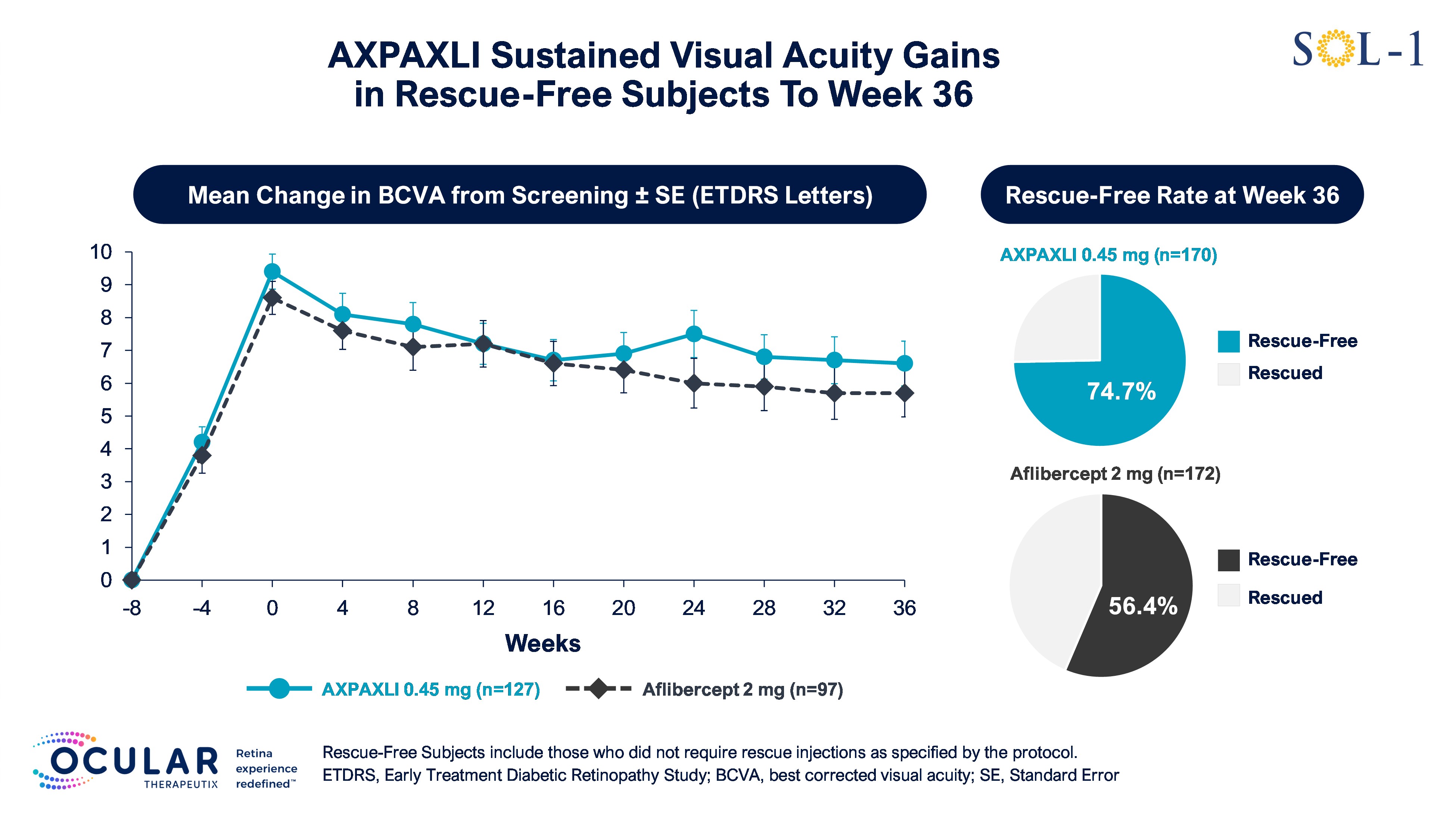
Figure 6: Mean Change in CSFT in Rescue-Free Subjects from Screening to Week 36
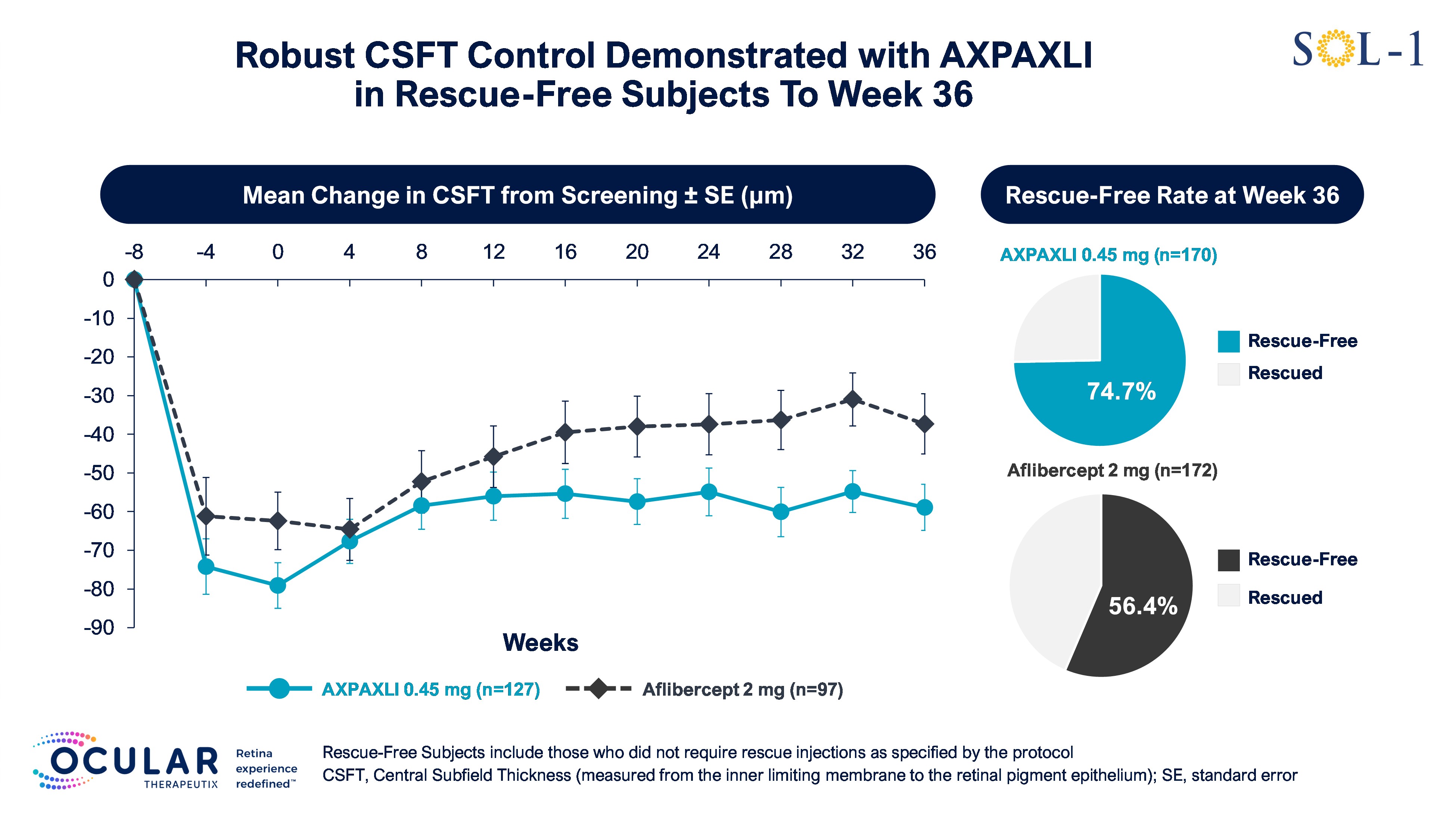
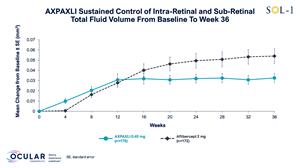
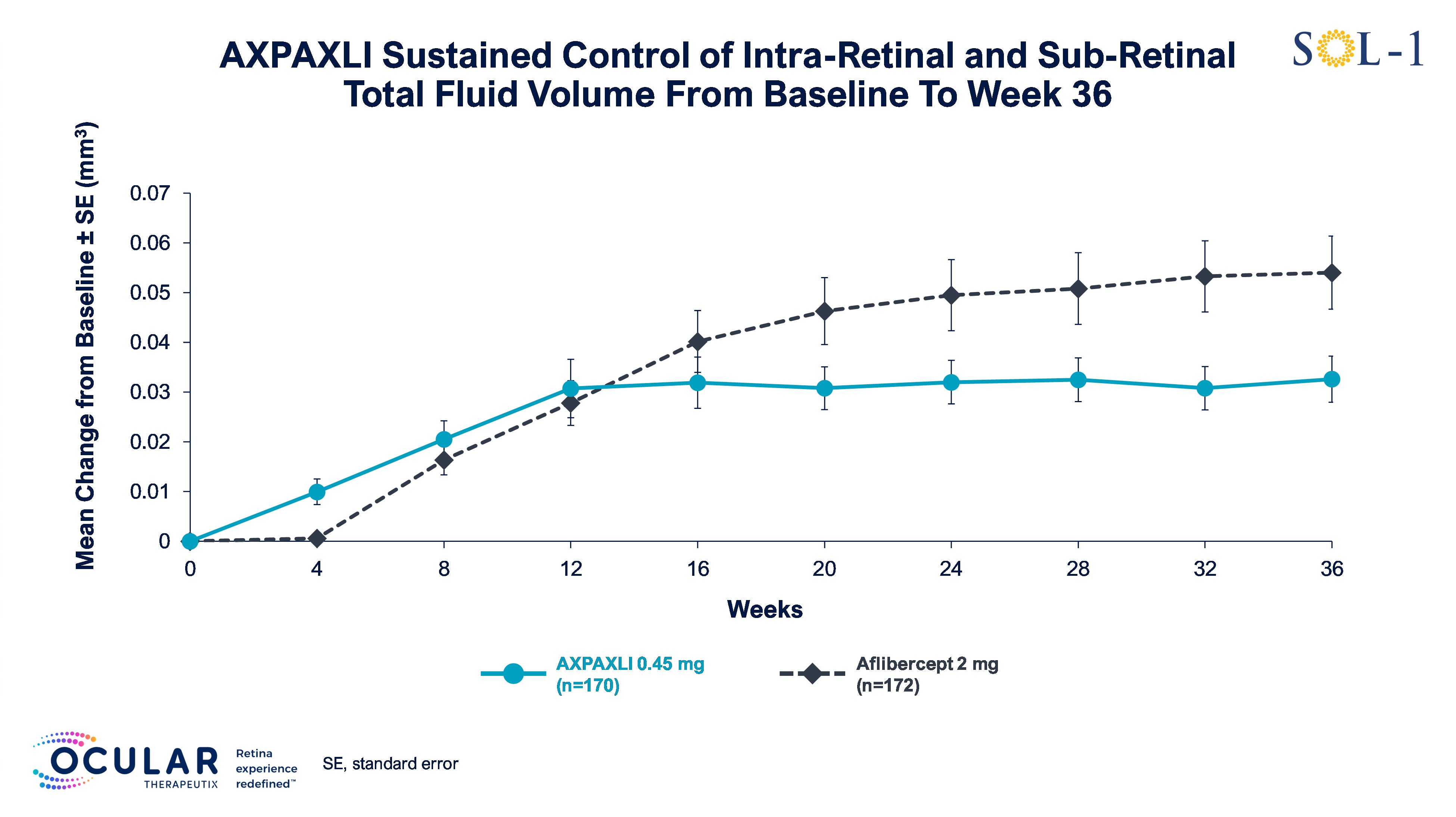
Figure 7: Total Fluid Volume in All Subjects from Baseline to Week 36
Infographics companying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/bfb14b75-6124-44ee-a517-06da07058760
https://www.globenewswire.com/NewsRoom/AttachmentNg/48ed465f-c0eb-480b-b11d-f6fe7d2fb84f
https://www.globenewswire.com/NewsRoom/AttachmentNg/210c6e3e-5c4f-42af-9755-6931116647c1
https://www.globenewswire.com/NewsRoom/AttachmentNg/73925d40-7ea6-4334-bd88-76a964e5fdde
https://www.globenewswire.com/NewsRoom/AttachmentNg/66648741-652e-4aea-900d-34ea6f5c6838
https://www.globenewswire.com/NewsRoom/AttachmentNg/67dad925-50b8-4c0d-ad91-579a52a24177
https://www.globenewswire.com/NewsRoom/AttachmentNg/07de9bca-f323-4d0d-b2a7-fdbc58ba9808

Figure 1
Screening and Baseline Ocular Characteristics
Figure 2
Safety Summary
Figure 3
Additional Safety Details
Figure 4
Proportion of Subjects with <15 ETDRS Letter Loss in BCVA from Baseline at Week 36 and Week 52
Figure 5
Mean Change in BCVA in Rescue-Free Subjects from Screening to Week 36
Figure 6
Mean Change in CSFT in Rescue-Free Subjects from Screening to Week 36
Figure 7
Total Fluid Volume in All Subjects from Baseline to Week 36
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.